Abstract
Introduction: Patient selection is a challenge in designing effective phase I clinical trials in hematologic malignancies. Potential candidates typically have relapsed or refractory disease, and have had poor response to multiple prior lines of therapy. Such patients are at risk for early death, which reduces both the scientific yield and the therapeutic value of clinical trials: patients whose disease has already entered a terminal phase, and who subsequently die within a few months, provide little meaningful data to advance the understanding of an experimental treatment, and likely derive little clinical benefit from that treatment.
Currently, eligibility criteria for most trials rely on ad hoc laboratory thresholds, and on subjective assessments of performance status and life expectancy. It is desirable to have an objective, statistically validated means of predicting survival, to optimize patient selection for phase I clinical trials. A prognostic scoring system for this purpose has been developed and validated for patients with solid tumors (Garrido-Laguna et al. Cancer . 2012;118(5):1422-1428); this model incorporates variables on tumor type and number of metastatic sites, which are not directly applicable outside of solid tumors. Similar work has recently been reported in hematologic malignancies (Benajiba et al. Anti-cancer drugs . 2017;28(5):540-545), using only three variables: performance status, serum albumin, and disease class. We developed a novel prognostic scoring system to estimate progression free (PFS) and overall (OS) survival for patients enrolled in phase I clinical trials for treatment of hematologic malignancy.
Methods: We retrospectively reviewed the electronic medical records of 106 patients enrolled in phase I clinical trials for treatment of hematologic malignancies at the UT Health San Antonio Cancer Center between 2000 and 2014. Descriptive, univariate, and multivariate analyses were performed to determine the parameters that carry prognostic significance. Cox proportional hazards regression models of Progression Free and Overall Survival (PFS, OS) were used for multivariate analyses. A backward elimination process with a relaxed P-value (0.2) threshold was used to select variables for adjustment.
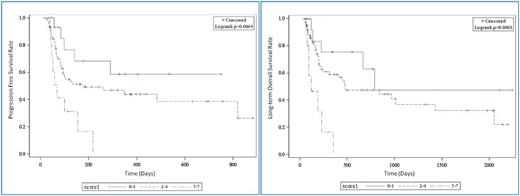
Results: Our analysis identified Hispanic ethnicity (P=0.03), low albumin level (<3.5 g/dL), low hemoglobin (<10 g/dL, P=0.02), hyperbilirubinemia (>1.0 mg/dL, P=0.03), abnormal white blood cell count (<4 k/µL or >10 k/µL, P<0.01), elevated lactate dehydrogenase (LDH) (>240 IU/L, P<0.01), and diagnosis of acute leukemia (P<0.01) as independent predictors of poor overall survival. The patient cohort was stratified by risk score into good (0-1), intermediate (2-4), and poor (5-7) risk categories, which were compared with respect to PFS and OS using Kaplan-Meier curves and log rank testing (see figures 1 and 2).
Conclusion: A simple score incorporating diagnosis, ethnicity, albumin, hemoglobin, bilirubin, white blood count, and LDH can identify patients with advanced hematologic malignancy who are likely to have short OS and PFS. Efforts are underway to validate this score against a separate cohort of phase I patients. Once validated, it could be incorporated in phase I trial design, potentially making these trials easier, faster, and more cost-effective to conduct, while allocating experimental treatments to those patients most likely to derive benefit.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.